FELIXprinters launches new food 3D printers series
February 7, 2022
Designer van dakpannen verkent mogelijkheden van 3D-printing
March 14, 2023MATMED FELIX BIOPRINTER
1. EXPERIMENT INFORMATION
| Project Description | The project aims to demonstrate and qualify a sacrificial bioink system and fully optimize it for FELIX Bioprinter dual-printhead module. This will allow the extension of commercial activities and broaden the portfolio by introducing a novel state of the art sacrificial material towards the printing of vascularized tissues and hollow structures. |
2. INTRODUCTION
One of the main challenges in tissue engineering is the lack of vascularization in the created tissue. Bioprinting has been one of the most promising approaches to generate high-resolution tissue models in a controlled manner. However, the printing of hollow structures and channels remains a challenge. One of the gold standards is the use of Pluronics as sacrificial ink to create such structures, yet its main drawback is that it only dissolves at cold temperature. Often larger structures also require to be placed on ice which is a limiting factor for cellular applications. This project will focus on the co-development and characterization of a sacrificial bioink which can be removed at physiological conditions in the presence of cells. The sacrificial ink will be combined with commercially available biocompatible bioinks and will be optimized for dual-printed bioprinting on the Felix Bioprinter. In this respect, the optimal printing parameters will be determined by varying printing speed, temperature and applied pressure during printing and the development of the printed structures will be fine-tuned. The biocompatibility of the sacrificial bioink will be addressed by in vitro cell studies.
3. EXPERIMENT DETAILS
| Material | SUPPORT INX
STABLE INX |
| Cell type | HT1080 GFP-labeled cells
HFF cells |
| Device | FELIX Bioprinter |
| Sterilization | 30 min UV |
| Solvents, cell culture media and other substances used | DMEM high glucose (Sigma, RNBK4361)
FBS (Sigma, 0001643829) Penstrep (Sigma 0000129403) PBS |
| Assay method | · Fluorescent microscopy
· MTS assay |
First, the printing parameters were optimized for the FELIX Bioprinter. The selection of the best parameters was done by visually inspecting and selecting the best results. The main characteristics for a correctly printed sample are a defined grid, visible pores, consistently sized edges, and fully printed lines which don’t break up. The shear thinning nature of the bioinks SUPPORT INX and STABLE INX allows the straightforward processing for deposition printing.
Figure 1. Viscosity of the used bioinks at different shear rates.
Table 1. Optimal printing parameters of SUPPORT INX.
| Nozzle Size | Nozzle Type | Flow Speed | Infill speed | Infill % | Layer height |
| 25 G | Conical | 100 % | 100 % | 100 | 0.156 |
| 25 G | Cylindrical | 85 % | 150 % | 100 | 0.175 |
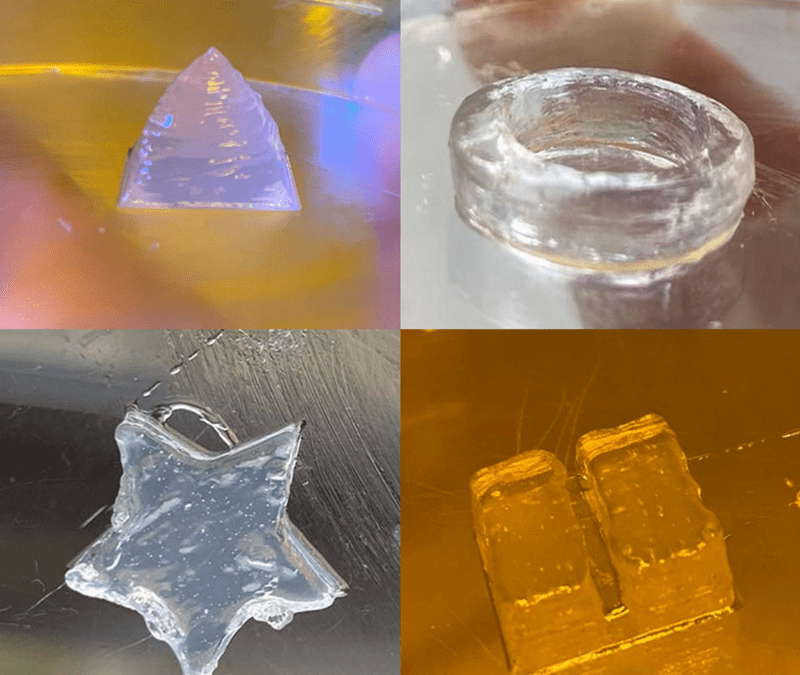
With the above-mentioned parameters structures with high shape fidelity could be printed.
Figure 2. Printed structures using SUPPORT INX.
Complex structures containing channels were successfully printed by combining STABLE INX with SUPPORT INX as support material. More specifically, 1 mm diameter straight and branching channels by flushing and applying a vacuum. Afterwards the scaffolds were sterilized and were seeded with GFP-labelled cells.
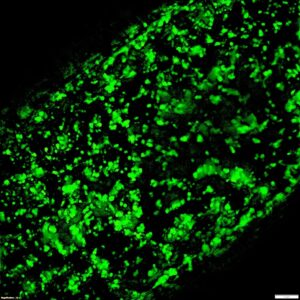
Figure 3. 1 mm in diameter channels were printed in STABLE INX using SUPPORT INX support followed by seeding with HT1080 cells.